Hydrogen energy is regarded as the most promising clean energy source in the 21st century due to its cleanliness, high heating value and abundance. There are various sources of hydrogen energy, and the traditional methods of hydrogen production include natural gas steam reforming, partial oxidation of hydrocarbons, and coal gasification etc. With the development of new energy sources, the use of solar energy and other renewable energy as raw materials to produce hydrogen on a large scale has become a common goal for all countries in the world. Figure 1 shows the conclusion of different hydrogen production methods.
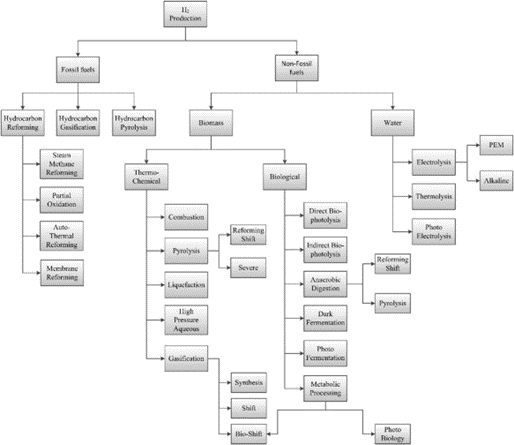
Figure 1 Hydrogen generation Pathway Demonstration (Milani et al., 2020)
At present, hydrogen production technology is becoming a prevailing topic of research in various countries. This article introduced the current research directions of green hydrogen production associated with solar energy, which includes solar thermal chemical cycle hydrogen production technology, photocatalytic hydrogen production technology and water split water hydrogen production by photovoltaic. Besides, the future development of solar hydrogen production technologies is also presented.
01 Solar Thermochemical Hydrogen Production
Solar thermochemical hydrogen production is the conversion of solar energy into chemical energy and the production of hydrogen from the decomposition of water through chemical reactions of metal oxidation. These include two processes: the absorption of heat by the decomposition of metal oxides and the liberation of heat from metal water. Compared to hydrogen production by pyrolysis of water, the solar-thermo-chemical cycle of hydrogen production not only overcomes the disadvantages of the direct method, such as the high temperatures required and the restrictions on the materials used for the reaction equipment, but is also much safer.
The solar thermochemical reaction cycle for hydrogen production is shown in Figure 2. Since H2 and O2 are not produced at the same time and in the same reactor, the problem of difficult separation of H2 and O2 at high temperatures is avoided and the reaction process allows water to be decomposed at a lower temperature, effectively reducing the requirements for the reaction conditions.
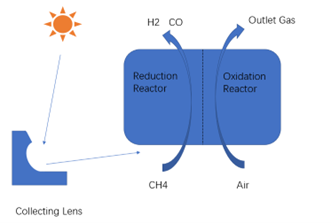
Figure 2 Schematic Diagram of Solar Thermochemical Reaction Cycle for Hydrogen Production
Although a lot of research has been done on solar thermal hydrogen production, low efficiency, high cost and high reaction temperature are still the challenges for this technology.
02 Photocatalysis for Hydrogen Production
When a semiconductor material is illuminated by the light with energy that higher than the band gap, the electrons in the crystal are excited to transition from the valence band to the conduction band, and free electrons and holes are formed in the conduction band and valence band respectively. Under the action of this electron-hole pair, water is ionized to generate H2 and O2.
Since Japanese scientists FUJISHIMA and HONDA first discovered the phenomenon of hydrogen production via photolysis on the electrode TiO2, the research of photocatalytic hydrogen production has become a popular topic of scientists around the world. With the development of the research, scientists have carried out doping and co-doping modifications on TiO2 through metal ions, non-metallic ions.
The doping of metal ions such as Fe3+, Co2+, Cu2+, Ni2+ and rare earth ions has improved the photogenerated charge lifetime, which in turn enhances the photocatalytic performance of the doped system. Non-metallic ion doping is the replacement or partial substitution of oxygen atoms in TiO2, which narrows the band gap of TiO2 to achieve a visible light response and makes TiO2 more hydrophilic, improving its surface adsorption capacity. Compared to metal ion doping and non-metal ion doping, co-doping improves the photocatalytic activity of TiO2 by extend its absorption for visible light.
03 Water Splitting to Produce Hydrogen
The application of solar energy has led to a gradual quantification of the process of water electrolysis and the evolution of 3 types of electrolyzers through continuous innovation and the use of selective features, which are alkaline electrolyzer, proton exchange membrane (PEM) electrolyzer and solid oxide electrolyzer cell (SOEC).
The alkaline electrolyzer was firstly invented, and it is the most widely used electrolyzer due to its technology maturity, low cost and simple process route.
The lifetime of the electrodes and the energy output are two key factors that need to be overcome in the use of alkaline electrolyzers, and therefore the use of nickel or nickel alloys with similar lattice properties as the activation coating for the cathode. A simple and effective electrodeposition technique has been used to prepare three-dimensional nickel-molybdenum electrocatalysts with controlled composition, size and nanoporosity on copper foams. The HER overpotential was reduced to 10 mV by optimizing the Ni/Mo ratio, electrodeposition current density and reaction time. With the continuous development and application of cathode materials, ternary and quaternary alloys such as Ni-Mo-P, Ni-Mo-N, Ni-Mo-Fe and Ni-Mo-P-B are gaining interest.
The PEM water electrolysis technique uses a proton selective permeation membrane to replace the membrane in a conventional alkaline electrolyser, which has an acidic effect in aqueous solutions, resulting in a higher oxygen absorption potential and reduced oxygen production, thus achieving high efficiency. Only precious metal electrodes such as platinum electrodes are suitable for this system, while other electrodes are prone to corrosion and therefore have high economic costs and are not suitable for industrial production.
In order to reduce the cost of PEM cells, researchers synthesized RuxPd1-xO2 bimetallic electrocatalysts using the Adams fusion method and prepared membrane electrode assemblies using 30% Pt/C as the anode and 30% Pt/C as the cathode, and investigated the electrochemical properties of the catalysts under different conditions. The results showed that the synthesized Ru0.8Pd0.2O2 electrocatalyst had a current density of 1 A·cm-2 at a cell voltage of 2.03 V at 80 °C and exhibited better electrocatalytic performance and stability than RuO2.
The Solid Oxide Electrolyzer Cell (SOEC) has received a great deal of attention since its discovery in 1972 and has been extensively investigated for the production of hydrogen from water treatment, particularly because of its high operating temperature (800-950 °C), conductivity and selectivity. Figure 3 is the Schematic diagram of SOEC.
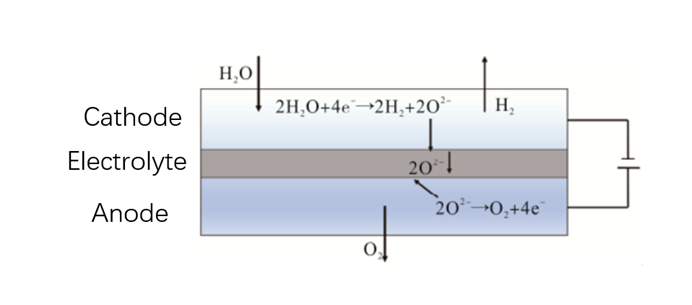
Figure 3 Schematic diagram of SOEC
Hydrogen is a renewable and green energy source that can solve both the energy crisis and the environmental pollution problem, and the wide application of hydrogen is the tendency toward the future. Although significant progress has been made in the production of hydrogen from solar energy, it is still required to further optimize and improve the production efficiency, material costs and scale of application of hydrogen production. By the improvement of cost performance of green hydrogen, it can be more competitive with traditional fossil fuel hydrogen production. The use of renewable energy to power the electrolysis of water to produce hydrogen is the most promising energy system of the future and the best route to a hydrogen economy. In the future, researchers should keep conducting further research on hydrogen production from electrolytic water.
MILANI, D., KIANI, A. & MCNAUGHTON, R. 2020. Renewable-powered hydrogen economy from Australia's perspective. International Journal of Hydrogen Energy, 45, 24125-24145.