The large-scale renewable energy promotion has put forward large-capacity and long-term storage requirements for energy storage systems. High-temperature Solid Oxide Electrolyzer Cell (SOEC) have become a hot spot in international research and development due to its high efficiency and reversibility, it has gradually become a representative of the "electric-hydrogen-electric" hydrogen energy storage technology route. From this perspective, this paper analyzes the development progress of using solid oxides water electrolysis for hydrogen production and energy storage technology.
01. Low Energy Consumption
SOEC electrolyzers operate at high temperatures (700-850°C), and the dynamics advantage of SOEC allows the use of inexpensive nickel electrodes. In addition, the high operating temperature can speed up the reaction rate of its electrolyzer electrodes, significantly reduce the overpotential of the cathode and anode, and effectively reduce the energy loss in the electrolysis process. If the high-quality waste heat in industrial production is utilized, the system efficiency of SOEC (LHV H2 to AC) could be up to 85%.
The principle of SOEC is the thermo-electric decomposition of water molecules, which has a significant advantage of low energy consumption compared with low-temperature ALK, AEM and PEM. The figure below shows the SOEC energy consumption assessment by the United States Department of Energy in 2021 and the comparison with PEM and ALK. The verified energy consumption and target energy consumption are marked in blue and green fonts respectively. Among them, the verified energy consumption of SOEC is 39kWh/kgH2.
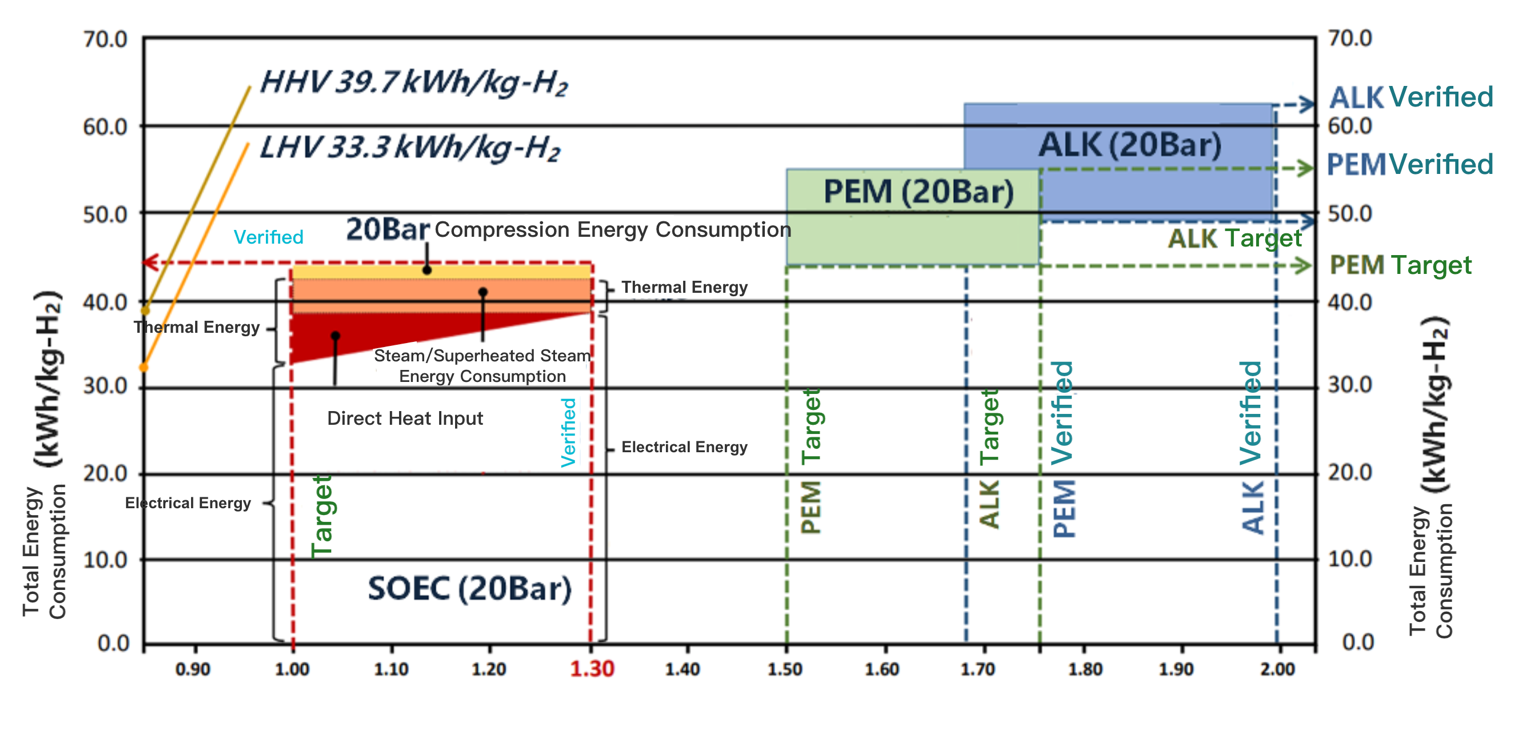
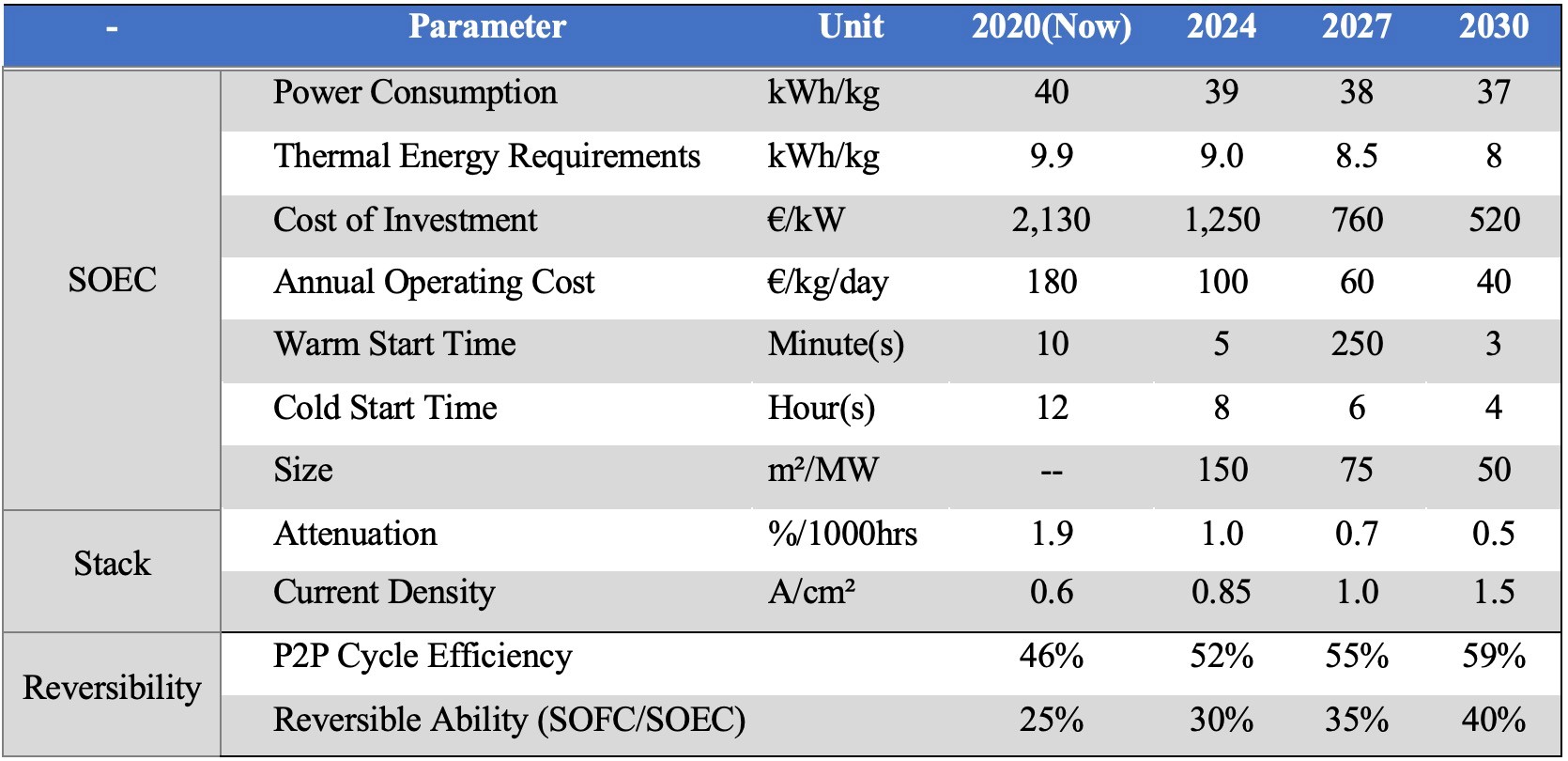
Another advantage of SOEC is reversibility, that is, Reversible Solid Oxide Cell (rSOC) are used for the storage of renewable energy to form an "electric-hydrogen-electric" hydrogen production, hydrogen storage and power generation system, which is also the long-term research and development focus in Europe and the United States. The table below shows the EU’s research and development goals for solid oxide water electrolysis, including the targets for P2P cycle efficiency (electricity-hydrogen-electricity) and reversibility to reach 52% and 30%, respectively, in 2024; and 59% and 40% respectively by 2030.
SOEC electrolyzers are fed with water vapour, and if carbon dioxide is added, syngas (a mixture of hydrogen and carbon monoxide) can be produced, which further produces synthetic fuels (e-fuels, such as diesel, jet fuel). Therefore, SOEC technology is expected to be widely used in carbon dioxide recovery, fuel production and chemical synthesis manufacturing, which is also the focus of research and development in the EU in recent years.
German Sunfire is the representative of European SOEC technology. The Saxony-based company was founded in 2010 and acquired a SOFC company the following year as the technological core of its later development. Based on a Power-to-Liquid (PtL) process, Sunfire built a 2.4MW SOEC demonstration project in the Netherlands in October 2020, producing 60kg of hydrogen per hour for synthetic fuel production, and its system electrical efficiency (LHV H2 to AC) target is 85%. The company secured a €109 million Series D round in November last year (it had previously raised more than €100 million) and plans to build 200MW of SOEC electrolyser capacity in 2023.
The EU's technical maturity assessment of its SOEC in 2020 is TRL7 (that is, the system prototype experiment has been completed in the operational, actual environment), which is higher than the United States Department of Energy's TRL5-6.
January 2020, the EU launched the SOEC demonstration project with a total budget of 9.75 million euros (of which FCH JU contributed 7 million), aiming to upgrade the technical maturity of SOEC from TRL 7 to TRL 8 within five years, and formulated the following key performance indicators:
- - System power consumption (standard working condition) ≤ 39kWh/kgH2
- - Stack decay rate ≤ 1.2%/1000 hours
- - Operating time ≥ 98 %
- - Investment cost per unit of production capacity (1 kg of hydrogen per day) ≤ EUR 2,400
- - Annual operating and maintenance costs (1 kg of hydrogen produced per day) ≤ 120 euros
05. Technological Progress
The technical difficulties of SOEC include stack degradation, system construction and safety. At the cell level, the degradation is mainly due to impurities in the input medium and structural changes inside the cell. At the stack level, in addition to cell performance degradation, degradation also comes from connectors, sealing materials, current collectors, etc. The factors that lead to the performance degradation of the stack are related to each other. Therefore, it is necessary to optimize the stack design, materials, and fabrication process from the perspective of the stack as a whole.
Durability is the primary issue of SOEC research and development at this stage. Thermochemical cycles, especially when the system is stopped and started, will accelerate components aging and reduce service life. Current solid oxide materials include zirconium dioxide, which is stabilized by adding 8% yttrium oxide, and its molecular formula is (ZrO2)0.92 (Y2O3)0.08. Improving the performance, durability and lowering operating temperature of solid oxides is currently the focus of research and development in Europe and the United States.
FuelCell Energy is a representative of American technology. The company oversaw a US$3 million SOEC R&D program from 2016-2020, and achieved the first three laboratory metrics below ahead of schedule.
- - Stack efficiency (LHV H2 to AC) > 95%
- - System efficiency (LHV H2 to AC) > 90%
- - System efficiency (LHV, calculated as electrical energy + thermal energy) > 75%
- - Single cell decay rate ≤ 1%/1000 hours; stack decay rate ≤ 2%/1000 hours
- - Develop subsystems to make SOEC compatible with intermittent renewable energy sources.
FuelCell Energy's 150-cell stack and system technical parameters in 2021 are as follows:
The following two figures show FuelCell Energy's stack production process and its 350-cell stacks for power generation, water electrolysis, and energy storage modes, respectively.
The bottom right of the figure is the energy storage system provided by FuelCell Energy for the J.M. Shafer Generating Station demonstration project in Fort Lupton, Colorado.
Mass production is currently the focus of research and development supported by the European and American governments. In January this year Sunfire and 15 partners led by it received 33 million euros in funding from the German Federal Ministry of Education and Research (BMBF) for SOEC electrolyzer system optimization, manufacturing processes upgrade and mass production.
In North America, Cummins acquired the solid oxide fuel cell business of General Electric (GE) in 2019, and received a $5 million grant from the United States Department of Energy in September last year for the research and development of SOEC stack automation assembly and production. The project will leverage Cummins' existing proven thermal spray method to automate the production of metal-backed solid oxide stacks, thereby reducing the costly sintering process and reducing the number of sealing parts required by 50%.
The three-year project with a total budget of US$ 7.2 million, completed independently by Cummins, aims to develop a standard prototype for the automated assembly of 60kW solid oxide stacks for the establishment of a SOEC electrolyzer plant with an annual capacity of 94MW.
The schematic diagram of Cummins thermal spraying method is shown below. The key technologies include durability of metal support; continuous, sequential additive manufacturing (3D printing); non-destructive real-time quality inspection, and traceability of each single cell.
The upper left of the figure shows the composition of a Cummins single cell: the middle is a dense electrolyte layer, and the two sides are porous hydrogen electrodes and oxygen electrodes. The main role of the electrolyte is to separate oxygen and fuel gas, also conducting oxygen ions, so the electrolyte is required to be dense and have high ionic conductivity with negligible electronic conductivity. The electrodes have a porous structure to facilitate gas diffusion and transport. In addition, Cummins flat-panel cells also require sealing materials, and stacks composed of hundreds of single cells also require connector materials (bipolar plates).
In addition to Oxygen-ion conducting conductors, O‐SOEC, Proton-conducting oxides (H-SOEC) is also a key research and development direction of the United States Department of Energy. As shown on the right of the figure below. H-SOEC is still in the early stage of research and development, and has the advantages of low operating temperature (550-750 oC), low activation energy, easy gas separation, and easy reversible operation of SOFC-SOEC; research and development difficulties include: slow kinetics, and that the electrolyte is not mature enough (e.g., synthesis, densification, H?conduction).
The figure below shows the stack cost estimates for O-SOEC and H-SOEC commissioned by the US Department of Energy in 2022. At this stage, the stack cost of the two technical routes is about $350/kW, while the BOP cost is about $900/kW. As the annual capacity reaches 1GW, the projected cost of the stack for O-SOEC and H-SOEC will drop to $115/kW and $78/kW, respectively.
Conclusion: In view of the large-scale promotion renewable energy and the high efficiency of SOEC electrolysis of water for hydrogen production, as well as the application of hydrogen as a clean fuel and industrial raw material in many fields, the maturity of SOEC hydrogen production technology (especially the breakthrough in material performance and durability) will bring broad application prospects for both narrow-sense hydrogen energy storage (“electricity - hydrogen - electricity”) and broad-sense hydrogen energy storage (“electricity - hydrogen”).